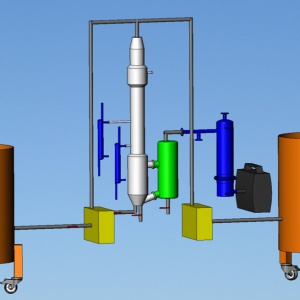
La unidad de elaboración está formada por elementos industriales que permiten producir numerosos tipos de cerveza. La instalación es modular y, para las necesidades de las recetas de sus cervezas, se puede utilizar según diferentes ciclos y procesos. El control es semiautomático, los elementos principales se controlan manualmente y a través de una pantalla táctil.
Los tanques principales de fábrica de cerveza, de filtración y de fermentación son de acero inoxidable 304L.
. Normalizar las recetas y los procesos de cerveza local
. Realizar el balance energético de toda una unidad de producción
. Estudio de esquemas de procesos, cadencias y ajustes
. Trabajar con una interfaz de supervisión de proceso
. Identificar los componentes de la instalación: instrumentación, actuadores, órganos de seguridad
. Estudiar los balances de energía y de materia de cada proceso
. Redactar notas e informes de funcionamiento que permitan la trazabilidad de los productos acabados
. Crear un sistema de análisis de riesgos de seguridad y gestión de la conformidad de los productos
. Un tanque de 25 l de acero inoxidable 304L de maceración, agitación y ebullición con anclaje de agitación y transferencia por bomba de impulsor blando
. Un cubo de filtración de acero inoxidable 304L de 50 L con fondo intermedio de chapa perforada de 25 L. Este cubo está estructurado como un filtro Büchner para facilitar la etapa de compresión de la masa en un filtro de bolsillo extraíble. La parte inferior está bajo presión reducida.
. 2 tanques de fermentación de acero inoxidable 304L de 25L equipados con una doble envoltura para mantener una temperatura de 10 a 25 º C, y elementos de seguridad adecuados para la fermentación. Un punto de punción en la parte superior del fondo cónico para no extraer el precipitado de la fermentación.
. Un intercambiador de placas, desmontable, de acero inoxidable 304L para enfriar rápidamente el producto después de la filtración. El intercambiador está conectado al sistema de refrigeración de los fermentadores.
. Un sistema de refrigeración con una potencia de refrigeración de aproximadamente 1,5 kW permite enfriar la mezcla en el intercambiador de placas y las dobles envolturas de los fermentadores.
. 2 bombas con impulsor, para efectuar transferencias entre depósitos. Los cabezales de bomba son de acero inoxidable 304L y los impulsores son de EPDM
. Una gama de instrumentos de medida que permiten la adquisición de datos: sondas PT100, caudalímetros electromagnéticos.
. Una variedad de elementos de seguridad: válvula, nivel alto y/o bajo,
. Un sistema de control y adquisición de datos en tiempo real se realiza a través de una pantalla táctil implantada en la caja de control principal. La interfaz permite visualizar los elementos de la instalación, los puntos de medición del proceso, los botones de control de los actuadores y la exportación de datos. Se muestran las alarmas y eventos del proceso. Se propone una página de parametrización para ajustar los parámetros del proceso de recetas.
. Un chasis de acero inoxidable 304L mecanosoldado con ruedas, dos de ellas con frenos
. Grupo de calentamiento para maceración, agitación y ebullición: termorregulador de 6 kW, agua presurizada de 110 ° C.
. Un molino de malta eléctrico (60 W) de 20 kg/h con cilindro de acero inoxidable para colocar sobre una mesa con un embudo de 4 L.
. Un sistema de limpieza instalado que utiliza bombas de proceso, en algunos casos, y una bomba sobre ruedas con 2 tanques de alimentación de HDPE de 60 L dedicados a los productos de limpieza y de aclarado.
. Conjunto automatizado en el que las válvulas manuales se sustituyen por válvulas automatizadas.
. Un sistema de supervisión remoto (a través de una red Ethernet) a través de un software dedicado para supervisar los datos de la instalación y para exportar los datos.